You have invested a lot in building your medical device business. Now, you want to take the next step and reach new countries to increase your market share but language barriers have become a limiting factor.
Of course, the last thing you want is a foreign language delaying your regulatory approval and hindering you from selling your product in the market. And that’s why medical device translation services are your most critical tool to navigate the linguistic and regulatory complexities you might face abroad across various markets.
In this blog post, we’ll share with you the key advantages of working with medical device translation experts. We’ll also discuss factors to consider when selecting a trusted partner.
What Do Medical Device Translation Services Cover?
Medical device translation is the process of translating and adapting medical device documents into multiple languages, making them accessible to healthcare professionals and patients in their native tongues.
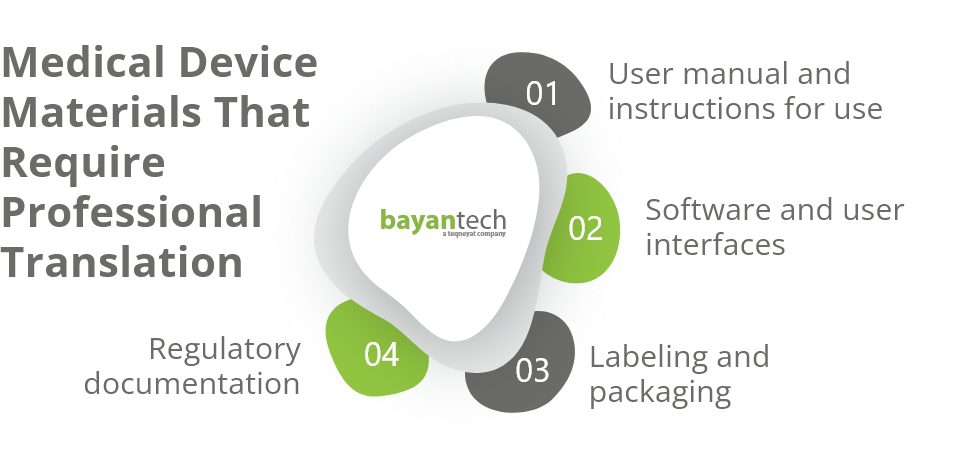
Let’s take a look at the different types of documents medical device translation services typically cover.
User Manuals and Instructions for Use (IFU)
IFUs and user manual translation provide essential safety information and usage instructions for end-users in their native tongues.
Software and User Interfaces
For medical devices that come with embedded software or eIFU (digital user manuals), localization services skillfully adapt software language and interface to maintain its usability and function in different languages.
Labeling & Packaging Materials
Medical device translation services also verify that all labels attached to the device and its packaging meet the target market’s requirements, ensuring that handling and storage instructions are easy to understand in the users’ language.
Regulatory Documentation
Medical device translators also work on all associated legal content, from registration documents to conformity declarations. They adapt all information accurately while maintaining compliance with the target country’s regulatory requirements.
The Important Role of Medical Device Translation Services
As a stakeholder, you may be wondering if hiring professional medical device translators brings value to your medical device manufacturing business and is worth the investment.
Well, to answer your question, consider this: Without expert translation services, expanding into new markets is not only complicated, but it could be entirely out of reach.
So, let us share with you three reasons why medical translation services are so important and how they streamline your entry into global markets.
Tapping into New Markets without Barriers
With professional translations, medical device companies can easily export their products to different countries and expand their market share without language barriers hindering their efforts. What’s more, translation services also assist in localizing your marketing materials. This helps in improving the appeal of medical devices to potential buyers, allowing you to penetrate your target markets successfully.
Maintaining Regulatory Compliance with International Laws
Life sciences and medical device industries are highly regulated sectors. Therefore, selling medical products in international markets requires adherence to strict local regulations imposed by various national bodies worldwide.
And most national authorities mandate medical device manufacturers to provide documentation in the country’s official language to market their medical products. So, without accurate and complete translations of these documents, this not only limits your market access but poses legal risks.
Ensuring Patient Safety and Device Effectiveness
Medical device translation also plays an essential role in providing users with important information in their native language, so you can ensure they know how to use the devices effectively. For instance, if the manuals are in a language that the user doesn’t understand, or if they’re poorly translated, it could lead to misuse or accidents, which exposes patients to risk and affects their overall safety.
3 Key Considerations For Medical Device Documents Translation
The complex and regulated nature of medical documents demands a highly experienced translation team equipped to handle the complexities of medical subjects.
So, how can you choose a reliable medical device translation service that checks all the boxes? Here are 3 factors to consider.
Technical Accuracy & Subject Matter Expertise
First, you should make sure the translation project is handled by qualified medical translators who are native-speaking subject matter experts. Assigned translators must have in-depth knowledge of specialized terminologies, quality standards, and medical regulations in the target language.
Rigorous Quality Control
Additionally, ask about their quality assurance processes, like proofreading and back-translation. This is very crucial to guarantee the integrity and accuracy of translated documents and verify their adherence to market regulations.
Confidentiality
Finally, consider the data protection procedures of the language partner of choice. Since medical content contains sensitive information, the translation process should be handled with strict confidentiality using platforms that limit third-party access. Also, make sure to sign an NDA before starting the project.
bayantech: Certified Medical Device Translation Services That You Can Trust
bayantech is a certified language service provider with two decades of experience serving clients from all around the world. We offer fast, accurate, medical translation services at a competitive rate and in compliance with ISO 17100 2015 and ISO 9001 2015 quality management systems.
At bayantech, we guarantee you 24/7 support and a team of native-speaking medical translators who are fluent in your target language and up-to-date with the industry’s strict requirements.
Contact us today and discuss your needs with one of our project managers.
8 Steps Every Medical Interpreter Takes
Looking for a medical interpreter? Discover the career path of medical interpreters and qualifications they need to acquire to take on interpreting jobs.